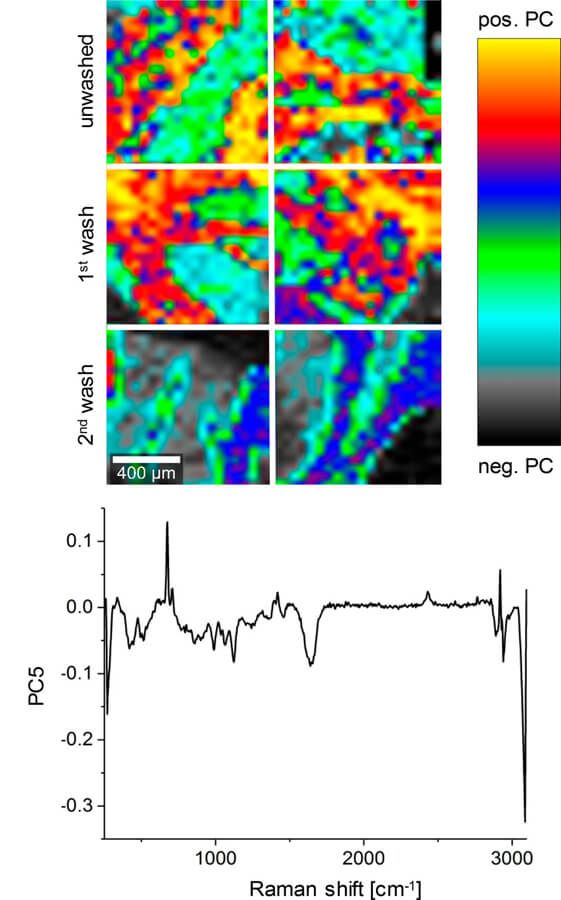
Cryomedium toxicity is a major safety concern when transplanting cryopreserved organs. Therefore, thorough removal of potentially toxic cryoprotective agents (CPAs) is required before transplantation. CPAs such as dimethyl-sulfoxide (DMSO), propylene glycol (PG), and formamide (FMD), routinely employed in ice-free cryopreservation (IFC), have advantages in long-term preservation of tissue structures compared with conventional cryopreservation employing lower CPA concentrations. This study evaluated the impact of potential residual CPAs on human cardiac valves. Raman microspectroscopy and Raman imaging were established as nondestructive marker-independent techniques for in situ quantitative assessment of CPA residues in IFC valve tissues. In detail, IFC valve leaflets and supernatants of the washing solutions were analyzed to determine the washing efficiency. A calibration model was developed according to the CPA's characteristic Raman signals to quantify DMSO, PG and FMD concentrations in the supernatants. Single point Raman measurements were performed on the intact tissues to analyze penetration properties. In addition, Raman imaging was utilized to visualize potential CPA residues. Our data showed that washing decreased the CPA concentration in the final washing solution by 99%, and no residues could be detected in the washed tissues, validating the multistep CPA removal protocol routinely used for IFC valves. Raman analysis of unwashed tissues showed different permeation characteristics depending on each CPA and their concentration. Our results demonstrate a great potential of Raman microspectroscopy and Raman imaging as marker-independent in situ tissue quality control tools with the ability to assess the presence and concentration of different chemical agents or drugs in preimplantation tissues.
ESC-derived cardiomyocyte differentiation
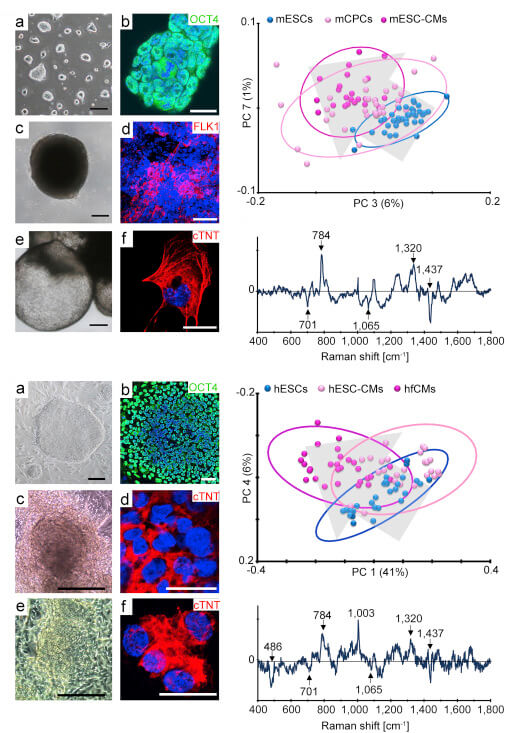
One major obstacle to the application of stem cell-derived cardiomyocytes (CMs) for disease modeling and clinical therapies is the inability to identify the developmental stage of these cells without the need for genetic manipulation or utilization of exogenous markers. In this study, we demonstrate that Raman microspectroscopy can non-invasively identify embryonic stem cell (ESC)-derived chamber-specific CMs and monitor cell maturation. Using this marker-free approach, Raman peaks were identified for atrial and ventricular CMs, ESCs were successfully discriminated from their cardiac derivatives, a distinct phenotypic spectrum for ESC-derived CMs was confirmed, and unique spectral differences between fetal versus adult CMs were detected. The real-time identification and characterization of CMs, their progenitors, and subpopulations by Raman microspectroscopy strongly correlated to the phenotypical features of these cells. Due to its high molecular resolution, Raman microspectroscopy offers distinct analytical characterization for differentiating cardiovascular cell populations.
Diagnostics - Disease in a Dish
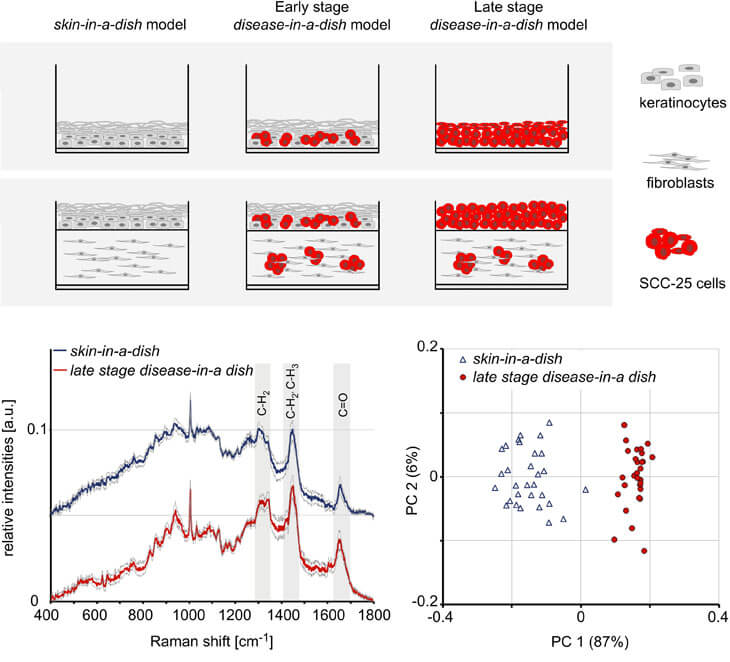
We introduced a commercially available squamous cell carcinoma (SCC) cell line SCC-25 into epidermal and full-thickness skin equivalents to generate human-based disease-in-a-dish model systems. Interestingly, when cultured either in the epidermis or dermis of full-thickness skin equivalents, SCC-25 cells formed hyper-keratinized tumor cell nests, a phenomenon that is frequently seen in the skin of patients afflicted with SCC. Raman spectroscopy was employed for the label-free cell phenotype characterization within the engineered skin equivalents and revealed the presence of differential protein patterns in keratinocytes and SCC-25 cells.