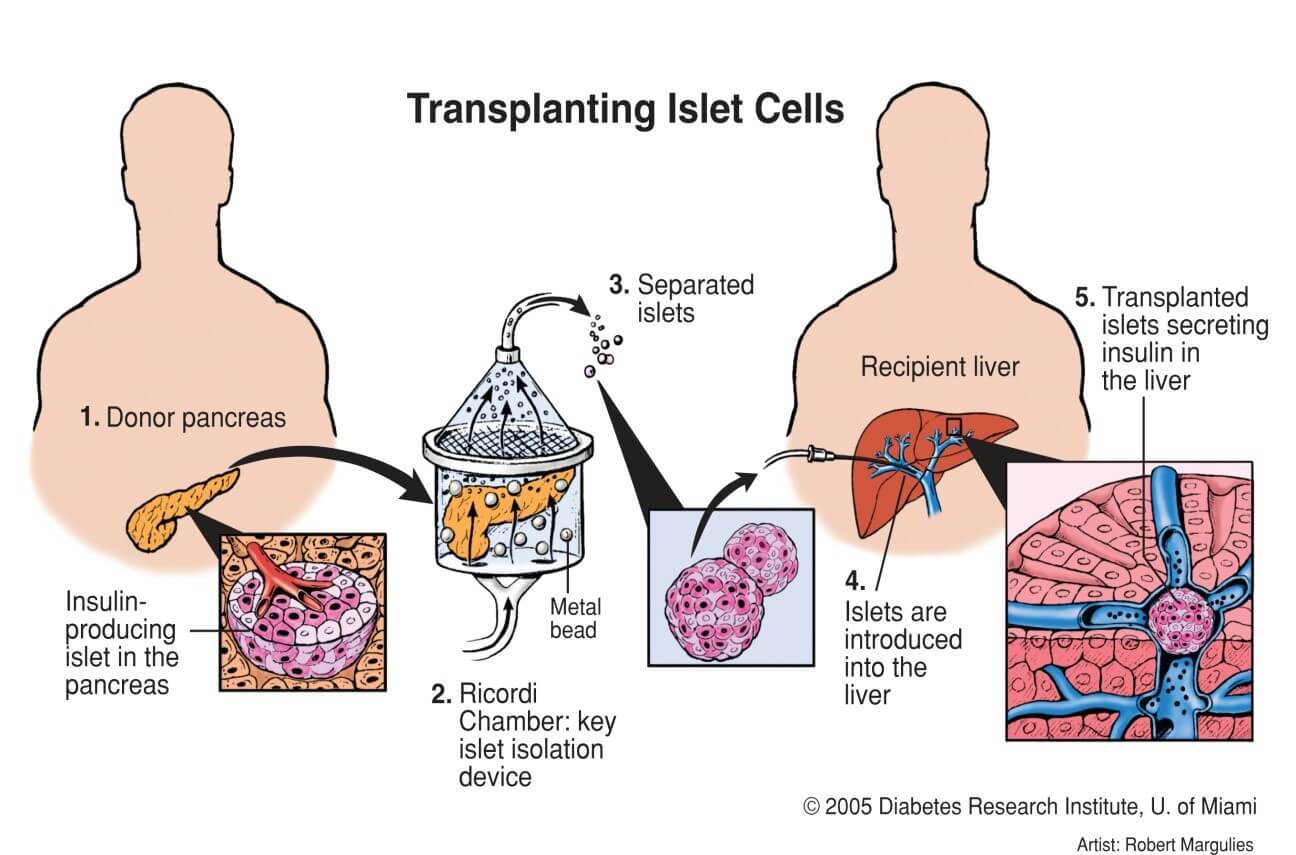
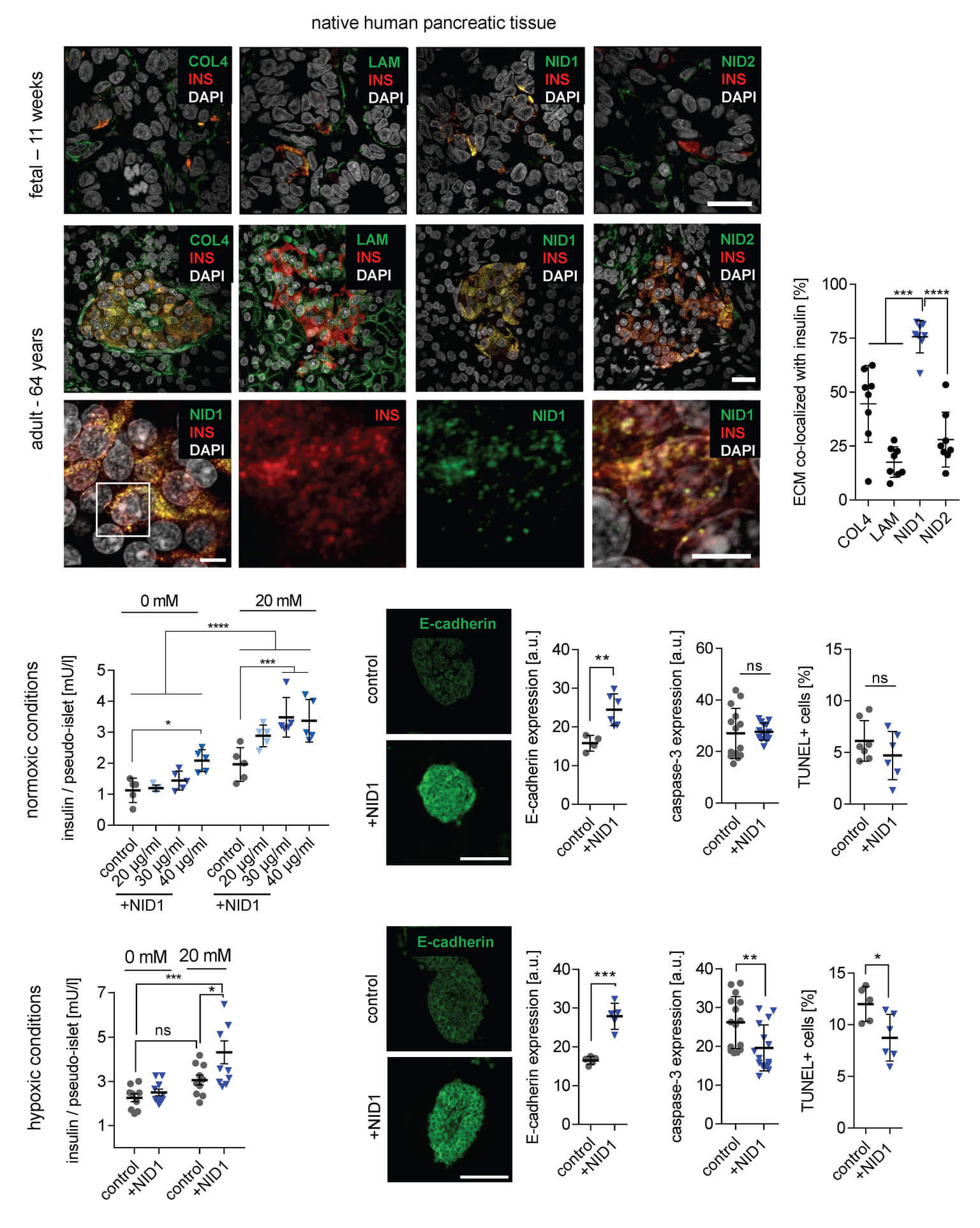
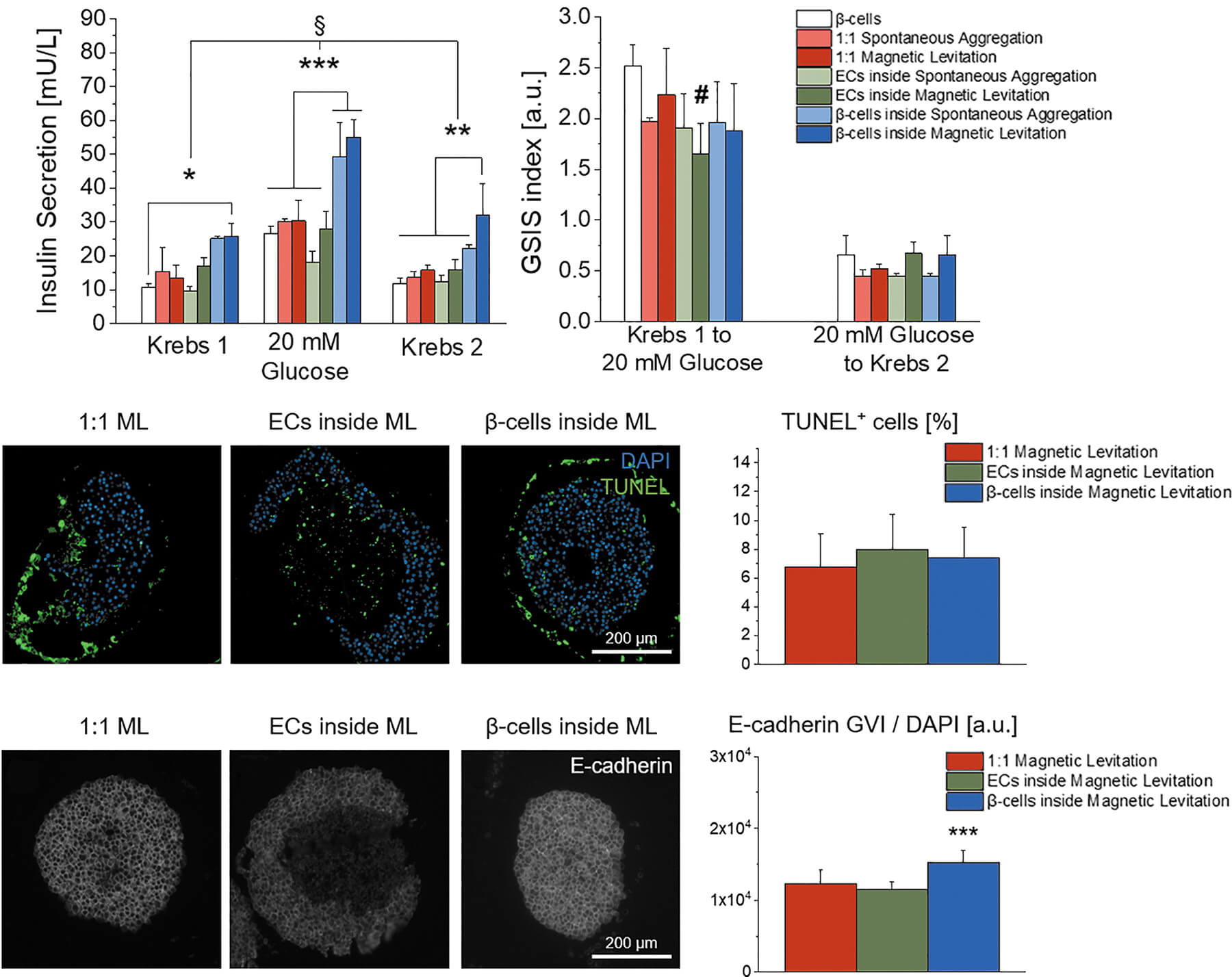
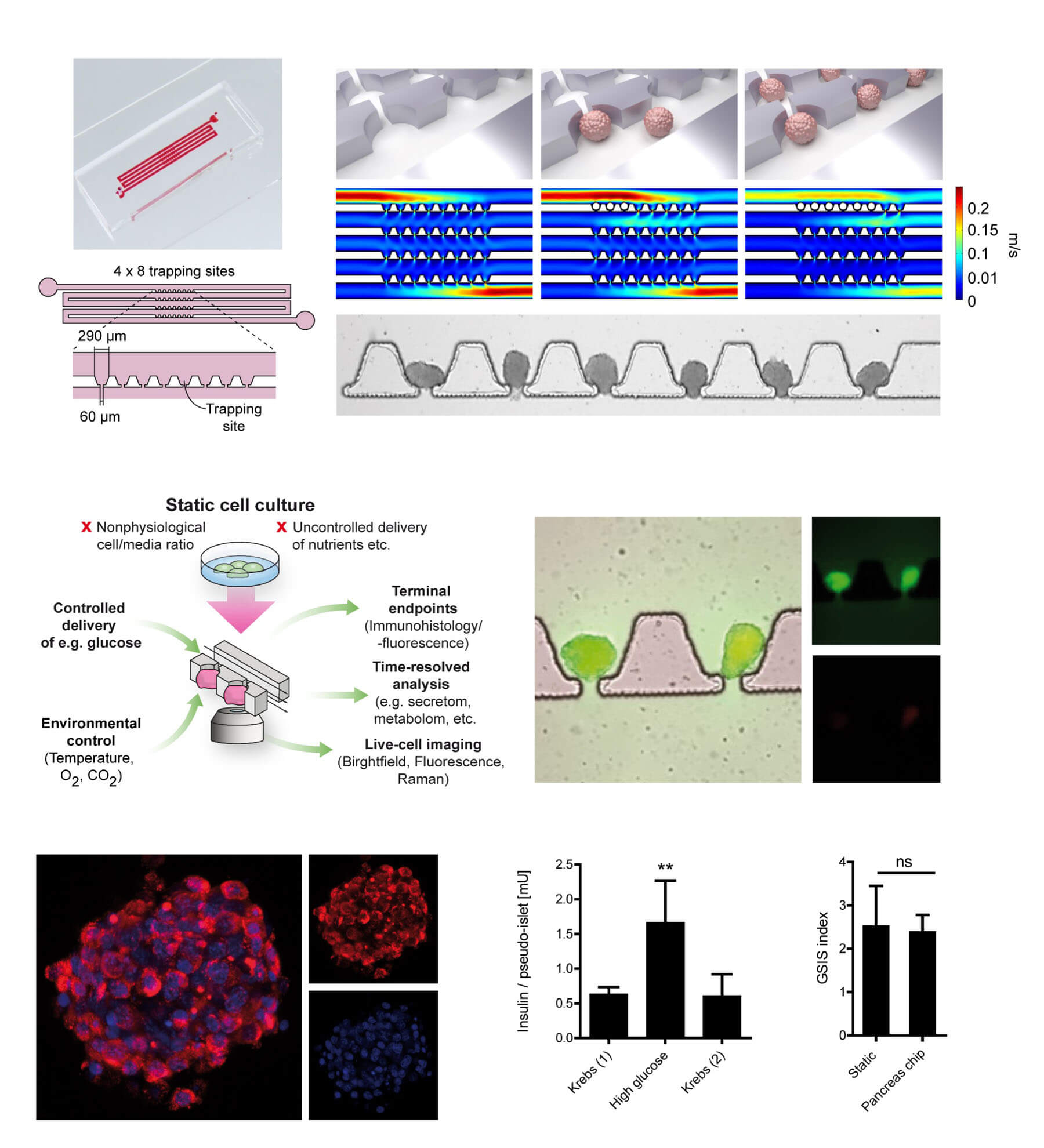
In a study published in Advanced Science, we demonstrated that Nidogen-1 (NID1) protects pancreatic β-cells and increases insulin secretion in an in vitro ischemia model, which mimics the conditions seen at an islet transplantation site. We tested the impact of dosages at 20, 30, and 40 µg mL−1 NID1 on pseudoislet function under normoxic conditions. NID1-treated pseudoislets significantly increased insulin secretion at all dosages; with 30 µg mL−1 reaching the maximum effect. IF staining of NID1-treated pseudoislets cultured under normoxic conditions showed a significant increase in E-cadherin when compared with the controls. NID1 had no effect on cell death under normoxic conditions, assessed by the quantification of TUNEL+ cells and cleaved caspase-3 staining. In hypoxic conditions, NID1 rescued the loss of insulin secretion that was lost in control cultures, preserved the significant increase in E-cadherin seen in normoxia, and significantly reduced cell death. We showed that NID1 binds and signals through integrin αvβ3 as seen by the upregulation of pFyn, Src, pSrc, and Rac1/cdc42, which leads to the activation of the mitogen‐activated protein kinases (MAPK) pathway, including the kinases extracellular signal‐regulated kinase 1/2 (Erk 1/2) and mitogen‐activated protein kinase 1/2.