Bioreactors are used to create biomechanical stress. In this project, we developed a bioreactor system to employ pulsatile flow (1.48 mL/min), cyclic strain (5%), and extended culture time to improve the maturation of murine and human ESC-CMs. Dynamically-cultured ESC-CMs showed an increased expression of cardiac-associated proteins and genes, cardiac ion channel genes, as well as increased SERCA activity and a Raman fingerprint with the presence of maturation-associated peaks similar to primary CMs. We presented a bioreactor platform that can serve as a foundation for the development of human-based cardiac in vitro models to verify drug candidates, and facilitates the study of cardiovascular development and disease.
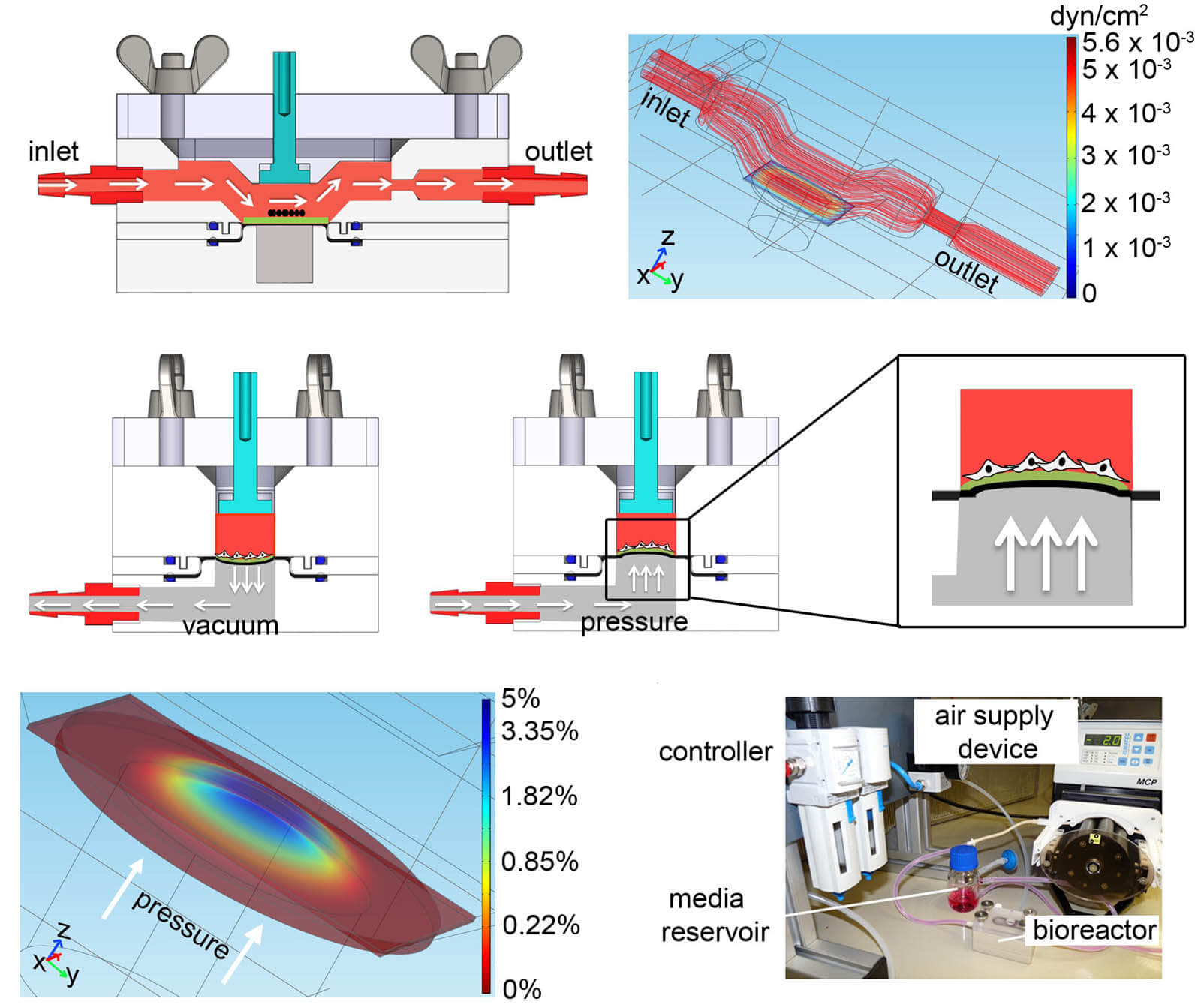
Bioreactors in cardiac engineering
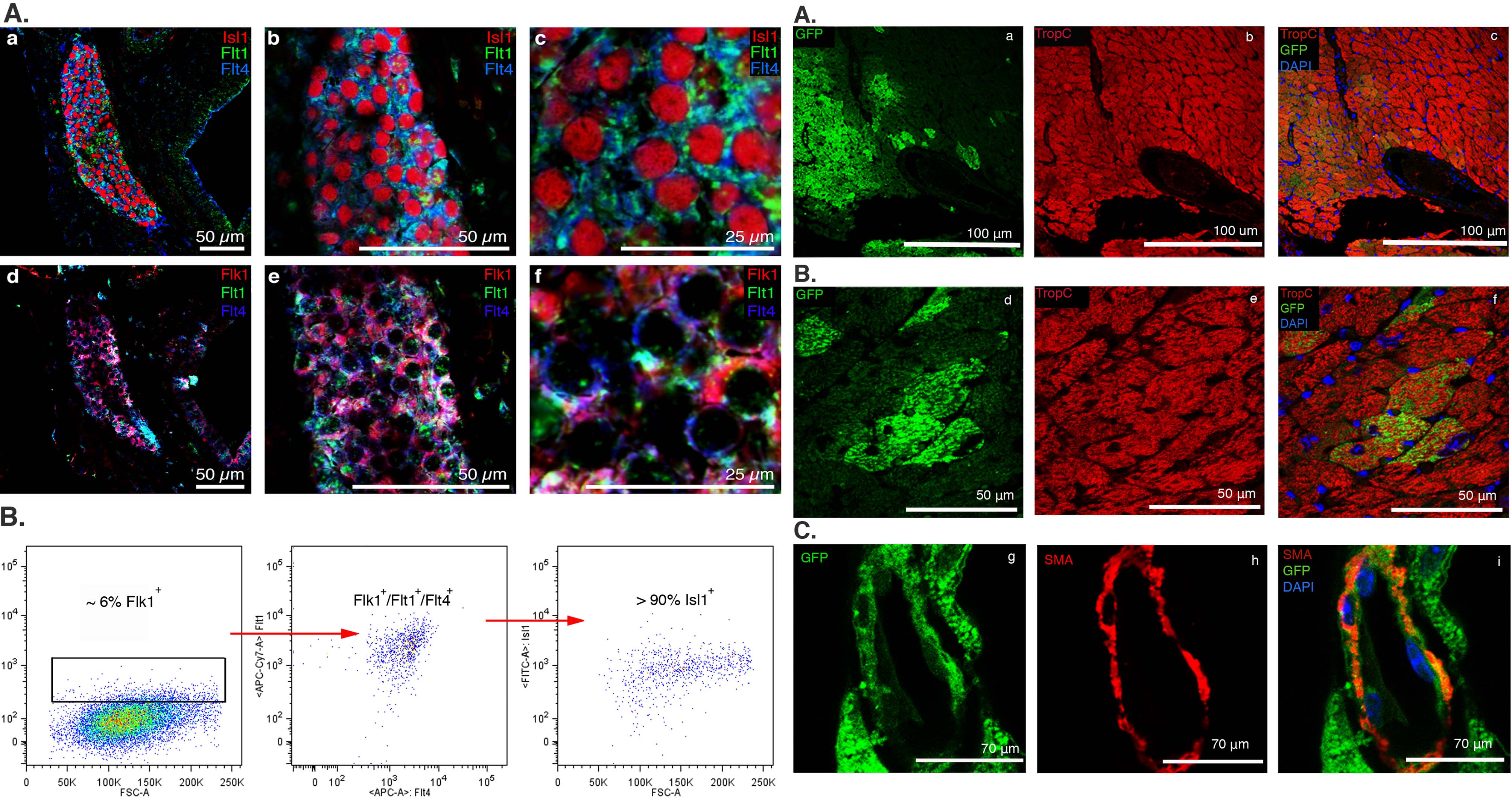
iPSC-derived cardiovascular progenitor cells
Cardiovascular progenitor cells (CPCs) have been identified within the developing mouse heart and differentiating pluripotent stem cells by intracellular transcription factors Nkx2.5 and Islet 1 (Isl1). Study of endogenous and induced pluripotent stem cell (iPSC)-derived CPCs has been limited due to the lack of specific cell surface markers to isolate them and conditions for their in vitro expansion that maintain their multipotency. We sought to identify specific cell surface markers that label endogenous embryonic CPCs and validated these markers in iPSC-derived Isl1+/Nkx2.5+ CPCs. We developed conditions that allow propagation and characterization of endogenous and iPSC-derived Isl1+/Nkx2.5+ CPCs and protocols for their clonal expansion in vitro and transplantation in vivo. Transcriptome analysis of CPCs from differentiating mouse embryonic stem cells identified a panel of surface markers. Comparison of these markers as well as previously described surface markers revealed the combination of Flt1+/Flt4+ best identified and facilitated enrichment for Isl1+/Nkx2.5+ CPCs from embryonic hearts and differentiating iPSCs. Endogenous mouse and iPSC-derived Flt1+/Flt4+ CPCs differentiated into all three cardiovascular lineages in vitro. Flt1+/Flt4+ CPCs transplanted into left ventricles demonstrated robust engraftment and differentiation into mature cardiomyocytes (CMs).
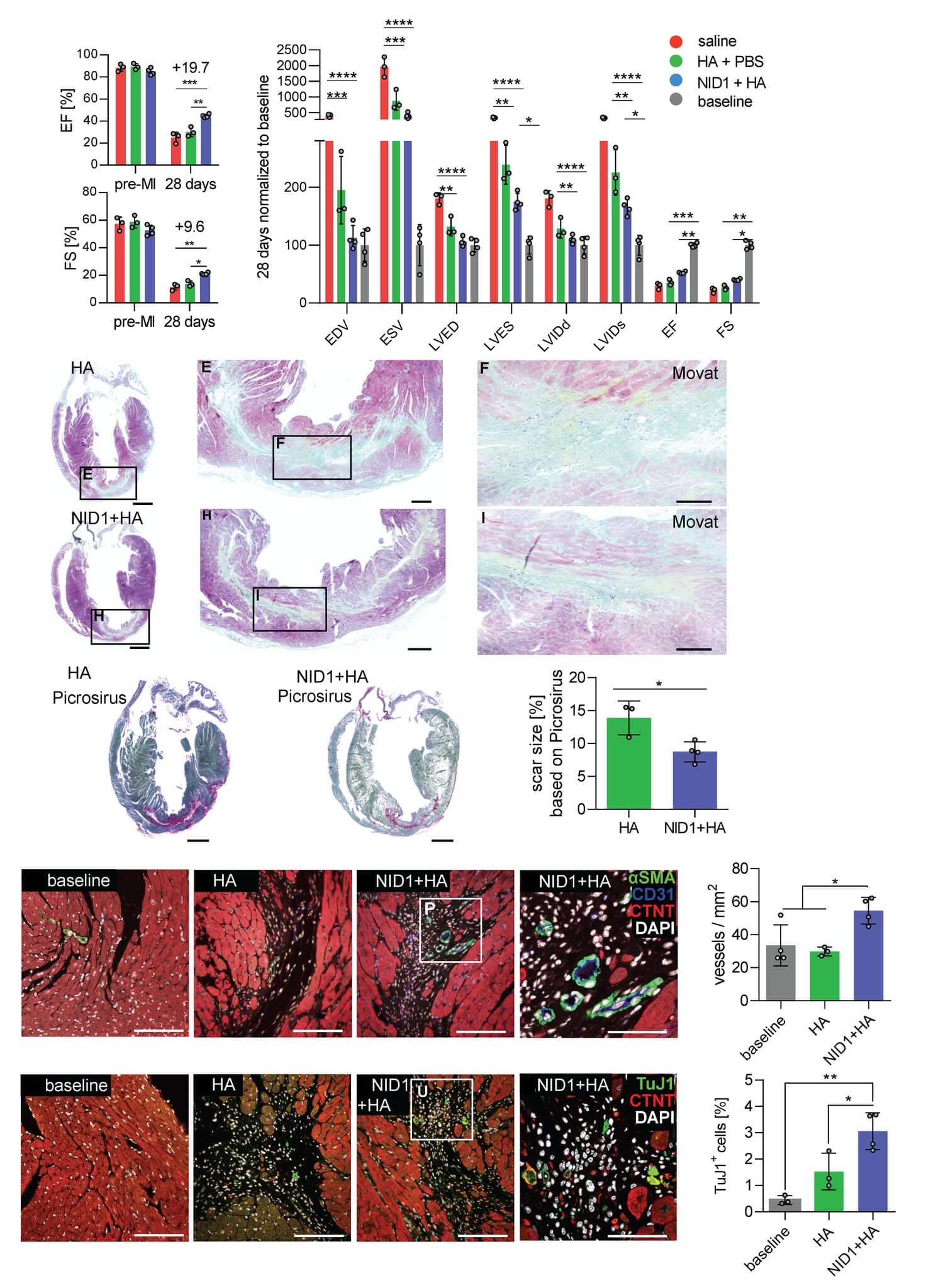
Nidogen-1 as a cardiac therapeutic candidate
Nidogen-1 (NID1) is a sulfated monomeric glycoprotein consisting of three globular domains: G1, G2 and G3 and with many epidermal growth factor repeats. It is an underinvestigated ECM basement membrane protein that is mostly recognized as a linker protein of COL4 and LAM. It has been suggested to play a role in angiogenesis, hepatic regeneration, and regenerative axon growth and guidance. Our research has demonstrated a potential cardiogenic effect of NID1 on differentiating pluripotent stem cells. We were further able to demonstrate that NID1 attenuates the apoptotic effect of hypoxia in cardiomyocytes and pancreatic beta cells via the αvβ3 integrin and beneficially modulates immune responses in vitro. We showed that NID1 significantly increases heart function and angiogenesis, while reducing fibrosis, in a mouse postmyocardial infarction model; therefore, demonstrating the protective and regenerative potential of NID1 in ischemic conditions.
Heart Valves
Currently available heart valve replacements are limited in long-term performance or fail due to leaflet thickening, lack of growth or remodeling potential. In order to address these issues, it is necessary to mimic multiple factors of the native valvular extracellular matrix (ECM) such as architecture, mechanical behavior and biochemical signals. In this work, we successfully generated an electrospun PEGdma–PLA scaffold adapted to the structure and mechanical properties of native valve leaflets. Valvular interstitial cells (VICs) and valvular endothelial cells (VECs) were seeded on the scaffold and when cultured under physiological conditions in a bioreactor, the construct performed like a native leaflet. Atomic force microscopy (AFM) was employed to obtain detailed mechanical information from the leaflets, which enabled the first layer-specific measurement of the Young's modulus. Interestingly, spongiosa stiffness was much lower compared to the fibrosa and ventricularis. Moreover, investigations into human fetal heart valve development identified collagen type I and versican as important structural proteins. As a proof of principle, these proteins were introduced to the scaffold, demonstrating the ability to bio-functionalize the hybrid valve based on natures' blueprint.
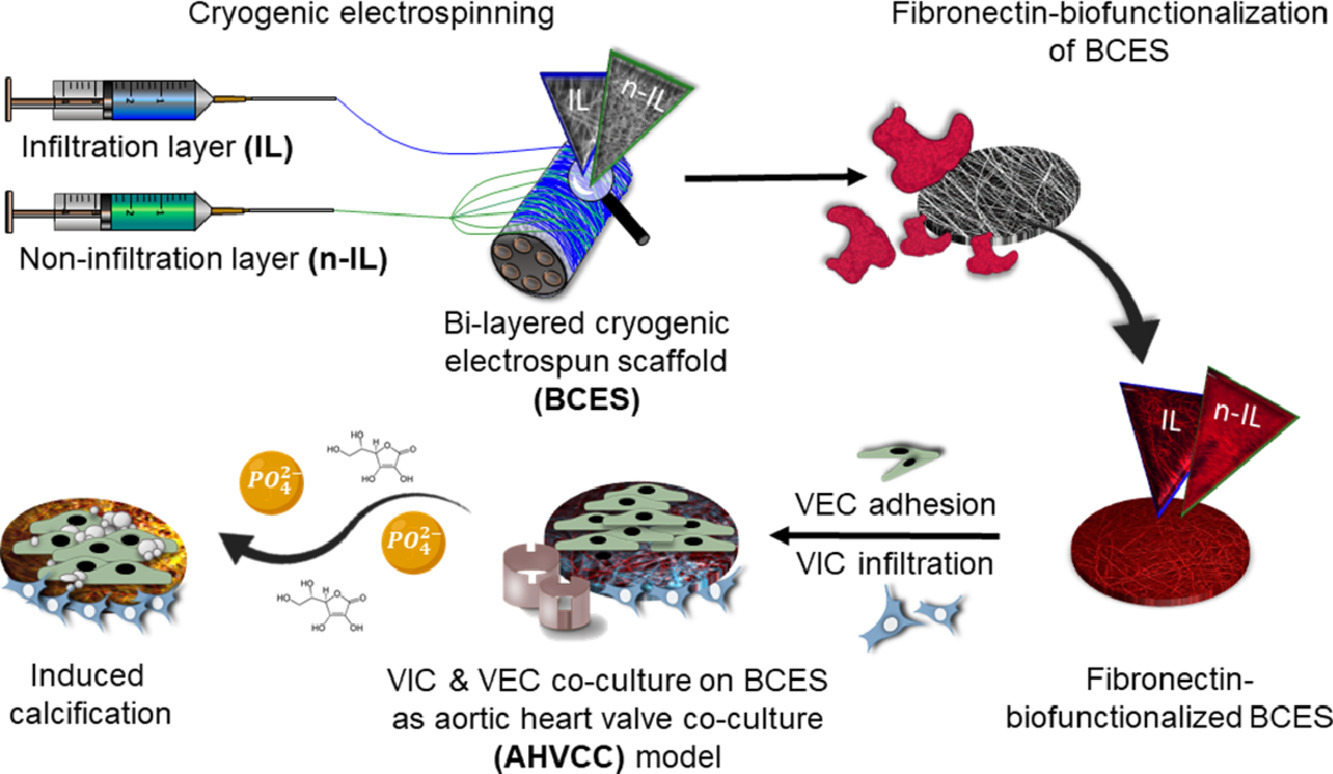
Heart valve disease models
Three-dimensional (3D) electrospun scaffolds are widely used for soft tissue engineering since they mimic the morphology of the native extracellular matrix. Several studies have shown that cells behave more naturally on 3D materials than on the commonly used stiff two-dimensional (2D) cell culture substrates, which have no biological properties. As appropriate 3D models for the study of aortic valve diseases are limited, we developed a novel bi-layered 3D in vitro test system by using the versatile technique of cryogenic electrospinning in combination with the influence of different solvents to mimic the morphology, mechanical, and cellular distribution of a native aortic heart valve leaflet. This 3D in vitro model can be used to study valve biology and heart valve-impacting diseases such as calcification to elucidate therapeutic targets.
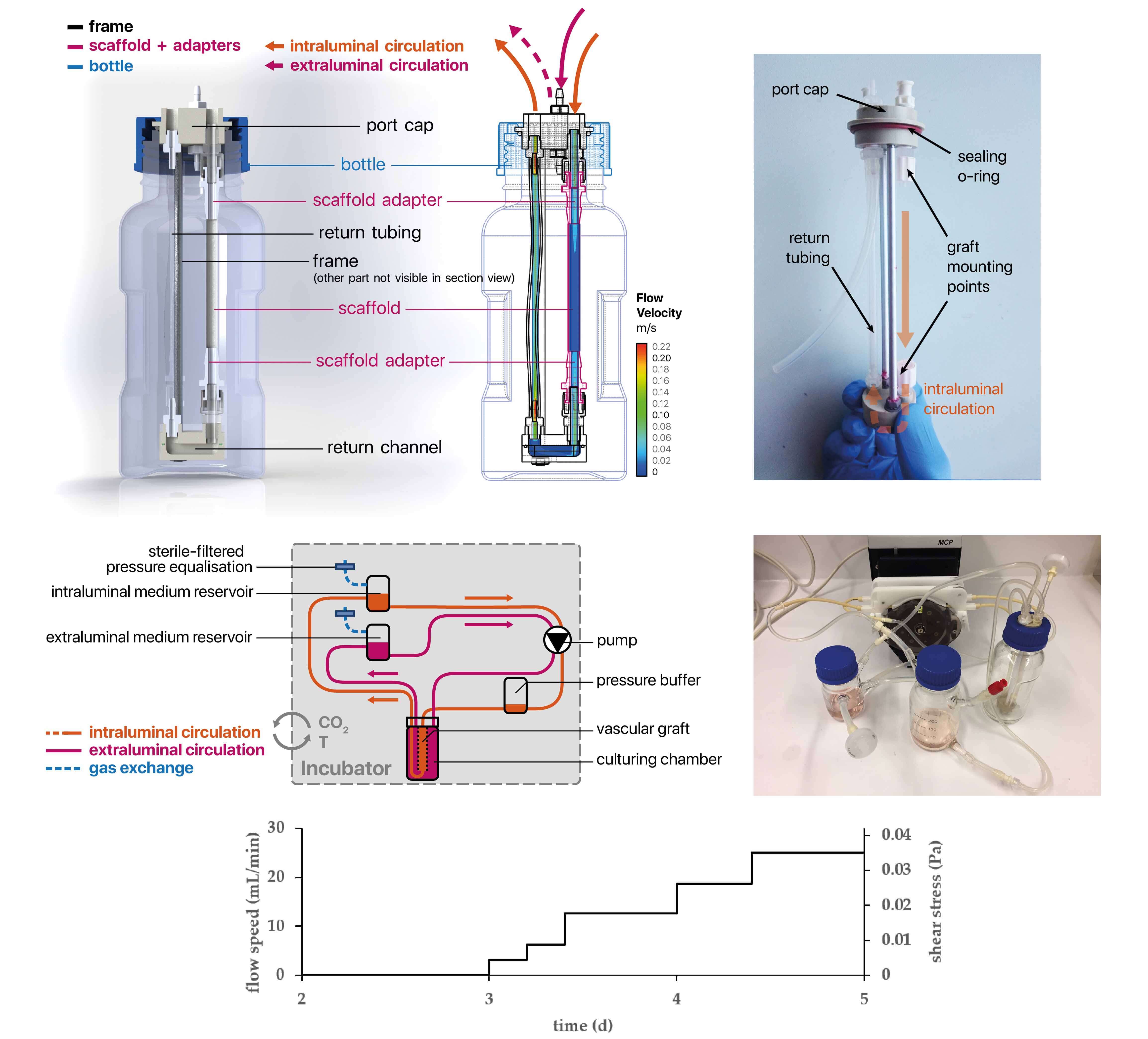
Blood Vessels
Appropriate mechanical properties and fast endothelialization of synthetic grafts are key to ensure long-term functionality of implants. In this study, we used a newly developed biostable polyurethane elastomer (TPCU) to engineer electrospun vascular scaffolds with promising mechanical properties (E-modulus: 4.8 ± 0.6 MPa, burst pressure: 3326 ± 78 mmHg), which were biofunctionalized with fibronectin (FN) and decorin (DCN). Neither uncoated nor biofunctionalized TPCU scaffolds induced major adverse immune responses except for minor signs of polymorph nuclear cell activation. The in vivo endothelial progenitor cell homing potential of the biofunctionalized scaffolds was simulated in vitro by attracting endothelial colony-forming cells (ECFCs). Although DCN coating did attract ECFCs in combination with FN (FN + DCN), DCN-coated TPCU scaffolds showed a cell-repellent effect in the absence of FN. In a tissue-engineering approach, the electrospun and biofunctionalized tubular grafts were cultured with primary-isolated vascular endothelial cells in a custom-made bioreactor under dynamic conditions with the aim to engineer an advanced therapy medicinal product. Both FN and FN + DCN functionalization supported the formation of a confluent and functional endothelial layer.